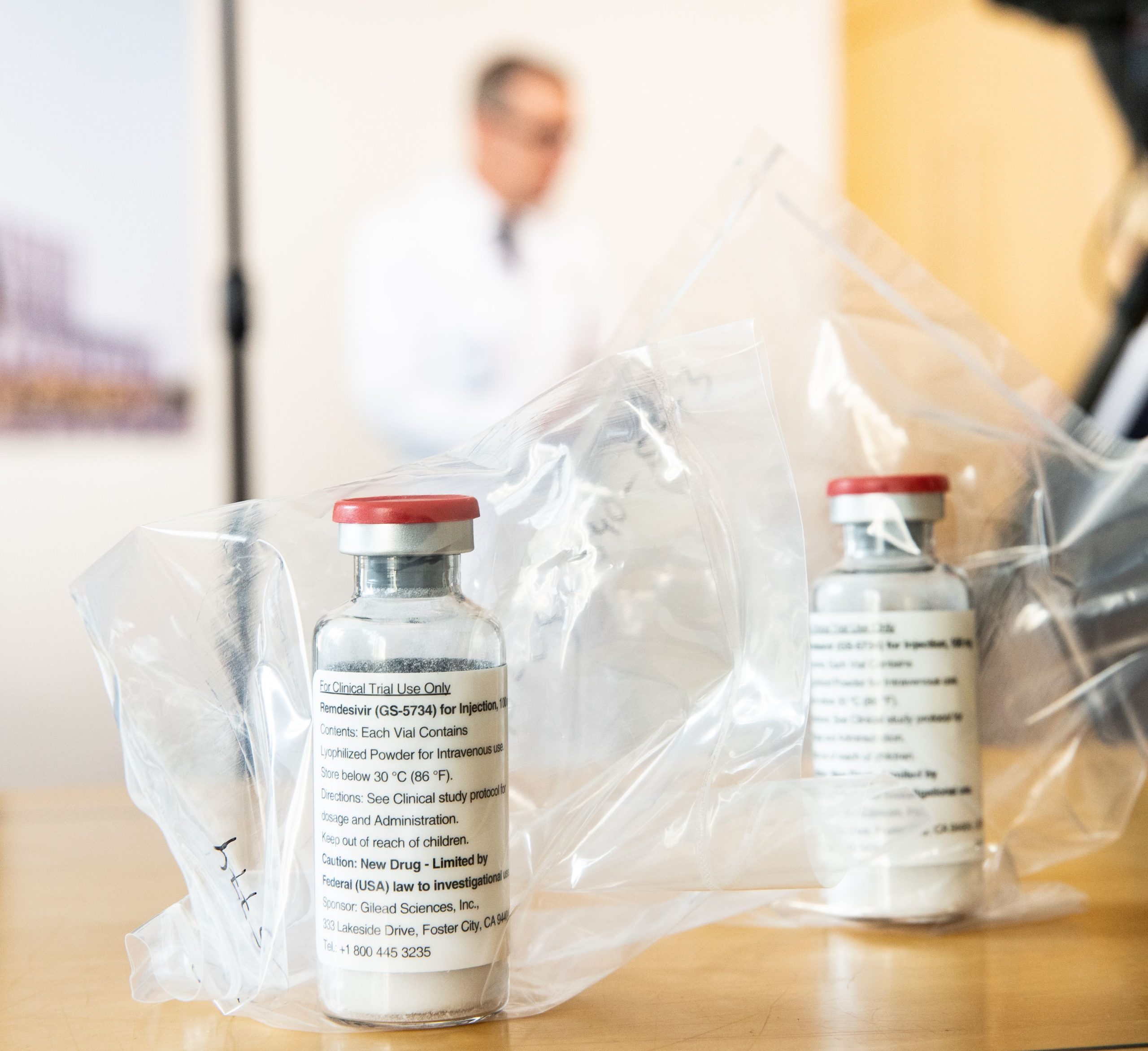
The European Medicines Agency (EMA) Thursday recommended a conditional marketing authorization for remdesivir as a COVID-19 treatment.
The EMA said remdesivir, produced by Gilead, can be used to treat patients over the age of 12 whose COVID-19 symptoms include pneumonia and who need oxygen therapy.
Conditional marketing authorization allows the EMA to recommend a medicine for early access in an emergency.
This means the medicine might be recommended with less data than a typical marketing authorization, provided that the agency determines that “the benefit of a medicines immediate availability to patients outweighs the risk,” the agency explained.
The Commissions decision, which is being fast-tracked, should follow “in the coming week,” according to a press release.
The EMA began a rolling review of remdesivir at the end of April, and it recommended expanding the compassionate use of the medicine on May 11.
The EMA based Thursdays recommendation on a study by the U.S. National Institute of Allergy and Infectious Diseases (NIAID). It showed that patients with severe COVID-19 symptoms recovered more quickly than those who received a placeboRead More – Source
[contf]
[contfnew]

politico
[contfnewc]
[contfnewc]